Is Ethanol a Covalent or Ionic Bond
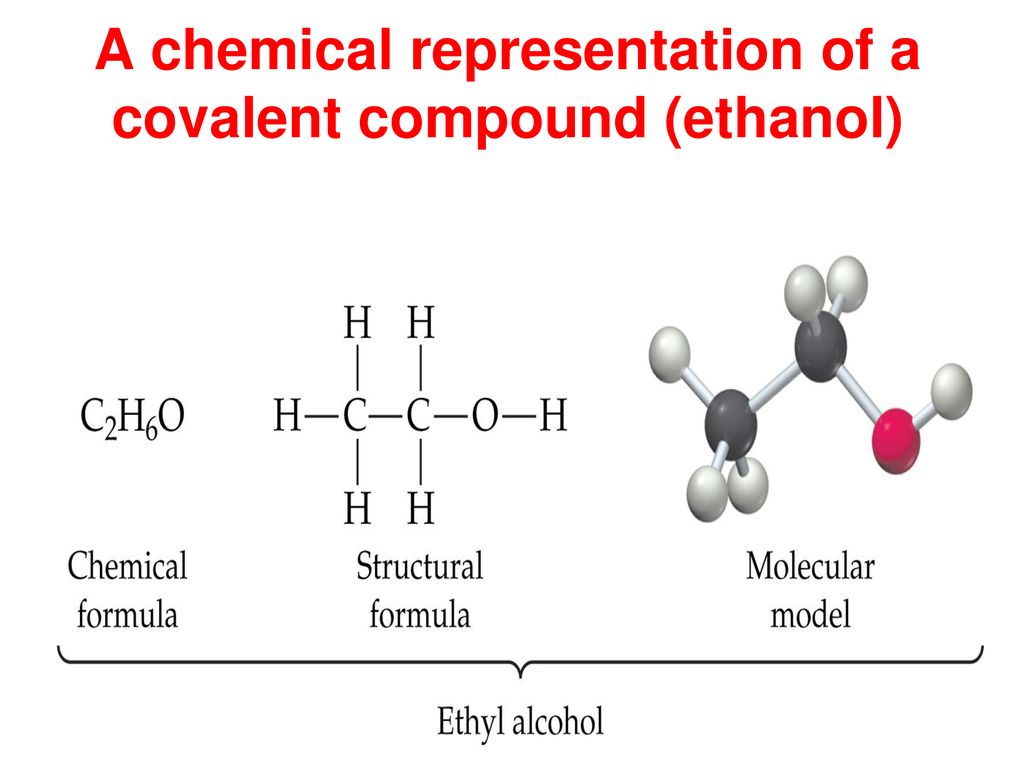
A common example is oil and water. Ethanol or C2H6O has two different types of bonding between its constituent atoms.
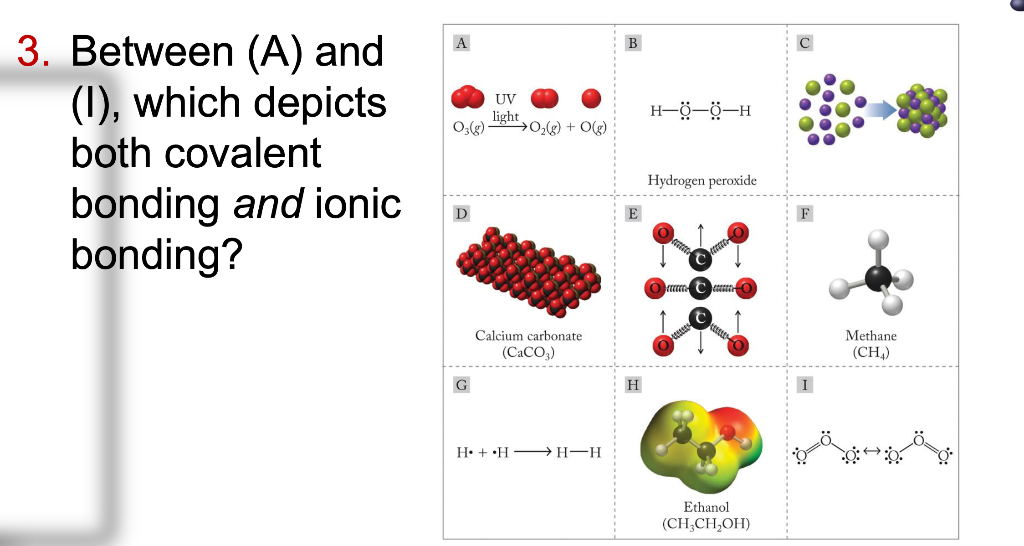
Solved C 0 G H 0 0 H 02 G O G 3 Between A And I Chegg Com
Is LiF soluble in ethanol.
. The hydrogen-oxygen and carbon-oxygen bonds are polar covalent bonds. The ammonium ion is polyatomic which means it forms ionic salts. Ethanol is a very polar molecule due to its hydroxyl OH group with the high electronegativity of oxygen allowing hydrogen bonding to take place with other molecules.
Being a polar substance it is a very good solvent itself. A chemical bond is a lasting attraction between atoms ions or molecules that enables the formation of chemical compounds. Ethanol or C2H6O has two different types of bonding between its constituent atoms.
Therefore whatever bonds it makes are ionic. Trace ionic character trend on the periodic table examine ionic vs. The hydrogen-oxygen and carbon-oxygen bonds are polar covalent bonds.
Thus it is a nonelectrolyte. These electron pairs are known as shared pairs or bonding pairs and the stable balance of attractive and repulsive forces between atoms when they share electrons is known as covalent bonding. Classify ethanol as an ionic or covalent compound.
What is chemical bond ionic bond covalent bond. For the first part of the question NH4CL contains both an iconic and covalent bond. The bonds between the hydrogen and carbon atoms are nonpolar covalent bonds.
The bonds between the hydrogen and carbon atoms are nonpolar covalent bonds. The bonding in the compound is all covalent so when isopropyl alcohol dissolves it separates into individual molecules but not ions. As there are no ions present in the formula of Ethanol an ionic bond can not be made.
Baing a covalent compound It does not conduct electricity because of the absence of free ions. BeBr2 is a nonpolar molecule as well. The molecular mass of ethanol is 46069 gmol1.
No electronegativity distinction between two atoms results in a pure non-polar covalent bond. Methanol CH3OH is a covalent bond. A small electronegativity distinction results in a polar covalent bond.
In ethanol C2H5OH 1 there are covalent bonds hydrogen bonds and van der Waals forces. There are kinds of aqueous solutions. The bond formed between any two atoms is not a purely ionic bond.
In a covalent bond electrons are shared between the two elements and will often favor one element over the other depending on polarity. Is ethanol an ionic substance. Ethanol therefore attracts polar and ionic molecules.
It is much easier to refer to ethanol as ethanol than to refer to it as the organic compound with two carbons six hydrogens and one oxygen that makes people drunk. All bonding interactions have some covalent character because the electron density remains shared between the atoms. Isopropyl alcohol is an organic molecule containing the alcohol functional group.
Covalent bonds and discover how to predict bond polarity. Is BeBr2 covalent or ionic. Ethanol or C2H6O has two different types of bonding between its constituent atoms.
Therefore a covalent bond occurs. In order for a compound to be considered an ionic compound remember that all compounds have both ionic and covalent properties the difference in electronegativity must be greater than 2. Does NH4CL have ionic and covalent bonds.
Ionic compounds are made of a metal plus a non-metal. To tell if C2H5OH Ethanol is ionic or covalent also called molecular we look at the Periodic Table that and see that C is a non-metal and O is a non-meta. The density of ethanol is 789 gl that is around 20 less than that of water.
For BeBr2 this condition is not met. Is ethanol covalent metallic or ionic. Lithium iodide for instance dissolves in natural solvents like ethanol not one thing which ionic substances usually do.
Nomenclature is not difficult but it istedious. We will start the exploration of nomenclature with simple covalent compounds and with ionic compounds. For sugar is C12H22O6 and Ethanol is C2H5OH can be used to tell if the bonds are ionic or covalent.
It is completely soluble in water. That said however the bond between N and H is covalent because both N and H are non-metals. Covalent compounds are made of non-metals combined with other non-metals 5 Using the Periodic Table explain how the position of the elements that make up the salts NaCl CaCl2 and KCl can be used to tell if.
For example C2H5OH ethanol is very soluble in H2O. 5 CH2Cl2 covalent bond. A covalent bond also called a molecular bond is a chemical bond that involves the sharing of electron pairs between atoms.
The bond may result from the electrostatic force of attraction between oppositely charged ions as in ionic. The bonds between the hydrogen and carbon atoms are nonpolar covalent. Oil contains molecules that are non-polar thus they do not dissolve in water.
Is Lithium a metallic. Ionic bonds are stronger than covalent bonds because the electronegativity difference between the two elements is much greater than that of two elements in a covalent bond. Both ionic and covalent bonds shared the fixed quantities during bond formation.
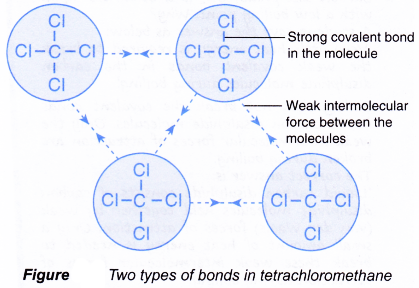
Properties Of Ionic And Covalent Compounds A Plus Topper
Illustrated Glossary Of Organic Chemistry Polar Covalent Bond

Chapter 7 Covalent Bonding And Lewis Formula S Ppt Download
Ionic Bond Test Dynamic Science
Are Ionic Compounds Soluble In Water And Not In Alcohol Why Quora

Bonding And Properties Ppt Download
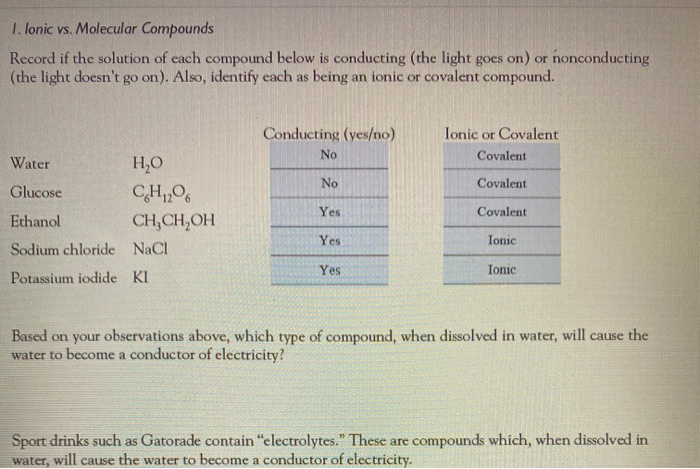
Solved 1 Ionic Vs Molecular Compounds Record If The Chegg Com

Hydrogen Bonding In Ethanol C2h5oh Youtube

Lab Identifying Chemical Bonds

Covalent Bond Of Ethanol Ethanoic Acid Carbon And Its Compounds Science Class 10

Chemical Bonds Forces That Attract Atoms To Each Other To Form Compounds Involves The Interactions Of Valence Electrons Between Atoms Usually The Ppt Download

Is C2h5oh Ethanol Ionic Or Covalent Molecular Youtube

Is C2h5oh Ethanol Ionic Or Covalent Molecular Youtube

Is Mineral Oil Polar Or Nonpolar Healing Picks In 2022 Covalent Bonding Ionization Energy Hydrogen Bond
Chemistry Rules In Brunei Year 11 Bonding And Structure Page
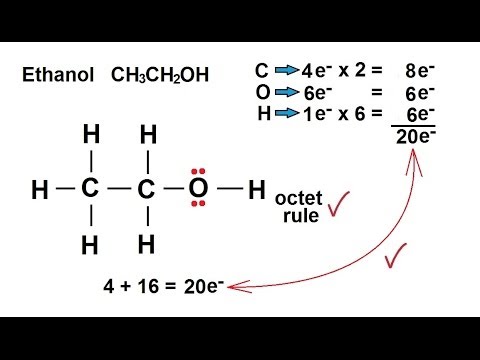
Chemistry Chemical Bonding 24 Of 35 Lewis Structures Ethanol Ch3ch2oh Youtube
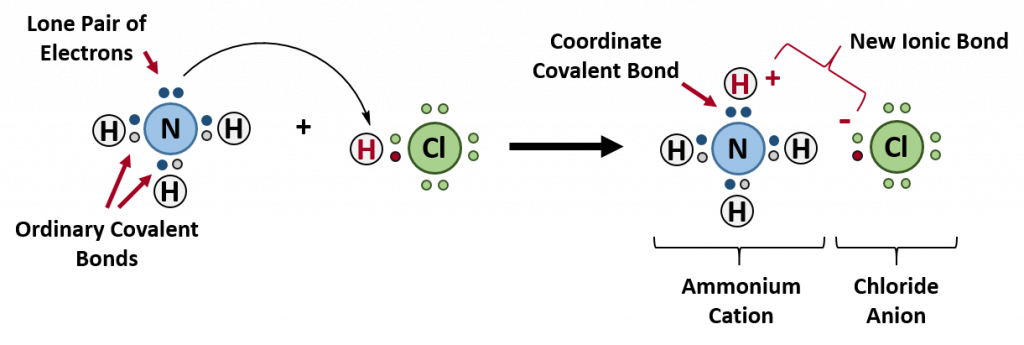
Ch103 Chapter 5 Covalent Bonds And Introduction To Organic Molecules Chemistry

Comments
Post a Comment